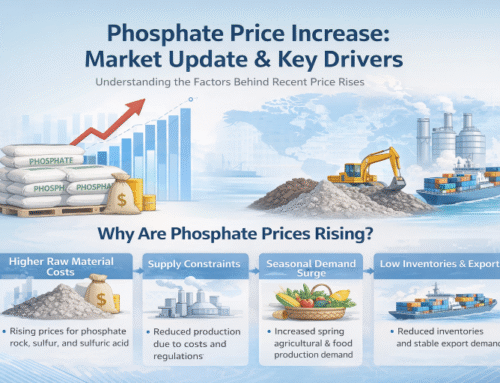
Introduction:
 Baking is as much a science as it is an art. Behind those delicious cakes, fluffy muffins, and mouthwatering bread lies a careful selection of ingredients that work in harmony to achieve the desired texture and structure. Two commonly used additives in bakery processing are sodium acid pyrophosphate (SAPP) and monocalcium phosphate, each with its distinct role. In this blog, we’ll delve into their differences and shed light on how they contribute to the magic that takes place inside your oven.
Baking is as much a science as it is an art. Behind those delicious cakes, fluffy muffins, and mouthwatering bread lies a careful selection of ingredients that work in harmony to achieve the desired texture and structure. Two commonly used additives in bakery processing are sodium acid pyrophosphate (SAPP) and monocalcium phosphate, each with its distinct role. In this blog, we’ll delve into their differences and shed light on how they contribute to the magic that takes place inside your oven.Sodium Acid Pyrophosphate (SAPP):
Sodium acid pyrophosphate, often known as SAPP, is an essential leavening agent in baking. Its primary function is to help the dough rise and enhance texture in various baked goods. When combined with baking soda, SAPP undergoes a reaction, releasing carbon dioxide gas that creates bubbles and causes the dough to expand. These gas pockets contribute to the light and airy texture of cakes, pancakes, and muffins.
One of the key advantages of SAPP is its fast-acting nature. It generates an almost immediate leavening effect, resulting in a quick and consistent rise in baked goods. This makes it an excellent choice for recipes that demand a rapid rise and a short baking time, allowing your cakes to achieve that perfect lightness and fluffiness.
Monocalcium Phosphate:
Monocalcium phosphate, commonly used in baking powder formulations, is another leavening agent that plays a crucial role in bakery processing. Unlike SAPP, it provides a slower leavening effect, gradually releasing carbon dioxide gas during baking.
The reaction of monocalcium phosphate occurs when it comes into contact with moisture and heat, triggering the release of carbon dioxide gas. Because of its delayed action, monocalcium phosphate is ideal for recipes that require an extended leavening process. Biscuits, scones, and certain bread doughs often benefit from this gradual rise, resulting in a desirable texture and flavor.
The Role of Timing:
The key distinction between SAPP and monocalcium phosphate lies in their reaction rates and timing. SAPP acts swiftly, allowing the dough to rise rapidly, which is crucial for achieving the desired texture in certain baked goods. On the other hand, monocalcium phosphate provides a more controlled and measured leavening effect, allowing bakers to manipulate the rise and texture of their creations over a longer period.
Application and Recipe Considerations:
Choosing between SAPP and monocalcium phosphate depends largely on the desired outcome and the specific recipe requirements. Depending on the nature of your baked goods, the selection of the appropriate leavening agent can greatly impact their final texture and structure.
For instance, if you’re baking delicate cakes or fluffy pancakes, SAPP would be the go-to choice, ensuring a quick and efficient rise to achieve the desired lightness. On the contrary, if you’re preparing biscuits or scones, where a delayed rise is necessary to create a flaky and tender texture, opt for monocalcium phosphate.
Conclusion:
Sodium acid pyrophosphate (SAPP) and monocalcium phosphate are indispensable ingredients in bakery processing, contributing to the magic that happens in the oven. While both serve as leavening agents, they differ in terms of reaction rate and their impact on the texture and structure of baked goods.
Understanding the role of SAPP and monocalcium phosphate allows bakers to make informed decisions when it comes to selecting the appropriate leavening agent for their recipes. By mastering this aspect of baking science, you’ll be well-equipped to achieve the perfect texture, flavor, and rise in your baked creations. Happy baking!